How The Jews Beat Tay Sachs
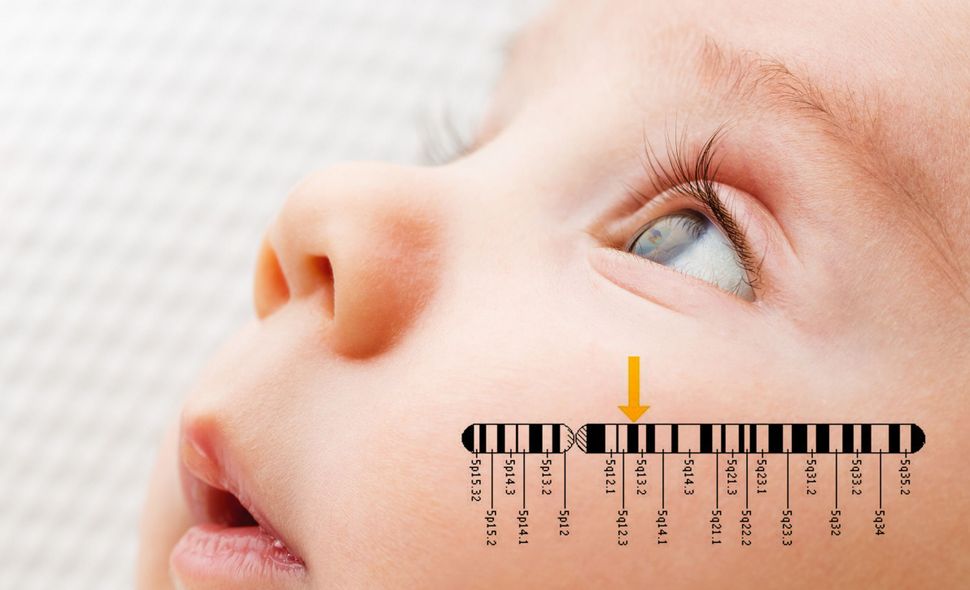
Image by Montage Kurt Hoffman
Not long ago, having a baby was a fairly straightforward venture. When a couple decided to have a child, they’d ditch the birth control pills and dim the lights. But with no plastic wand with twin purple lines to offer instant at-home confirmation, there was no easy way to gauge success.
The first home pregnancy test — a diagnostic tool now taken for granted by the 4 million women who give birth each year in the United States — wasn’t developed until the 1970s.
When I was conceived in 1971, my mother learned she was going to become a parent by heading to her doctor’s office after she missed her period. She was offered no genetic tests, got no real-time sonographic glimpse of her daughter-in-waiting, had no clue if I’d be a boy or a girl. She didn’t know if I’d emerge with ten fingers and the same number of toes, let alone the proper number of chromosomes. She was expecting, and she was oblivious to all the things that could go wrong.
Fast-forward four decades. We are a generation fueled by information, and at no time in our lives do we crave it more than when we are poised to become parents. We snap up pregnancy books and memorize developmental milestones as we steep ourselves in the minutiae of a world so unfamiliar it might as well be another country. Even the language is foreign — hyperemesis gravidarum? Reciprocal babbling? As we struggle to find our footing in this strange new place, the cluster of cells nested deep inside is already under scrutiny.
Now, not only do most parents know ahead of time what color to paint the nursery, but technology exists to tell them whether their developing cluster of cells has Down syndrome or a genetic deletion so tiny that it wouldn’t have been detected even a few years ago. Carrier screening techniques can now scan for hundreds of diseases. Mutations that heighten the lifetime likelihood of developing a great variety of diseases can also be identified, a dicey dance considering that many conditions, such as early-onset Alzheimer’s, cannot be treated: What good is it to know about a risk of disease if there’s nothing that can be done to help? This information exchange about possible or actual progeny is often taking place before the end of the first trimester, maybe even before an embryo implants, forcing parents to make difficult decisions based on an unprecedented deluge of data. Information is usually seen as a good thing, especially in this digital age. But is it possible to have too much information?
•
To imagine what the future of carrier screening might look like, it’s helpful to understand its history.
Michael Kaback was a young pediatric geneticist at Johns Hopkins in 1970 when, in the course of his clinical duties, he became deeply involved with two families. Harold and Bayla Gershowitz had a toddler son with Tay-Sachs disease. Kaback helped the Gershowitzes find care for Steven. The other couple, Bob and Karen Zeiger, also had a son, Michael, who was less than a year old. Bob Zeiger was a pediatric intern at Hopkins who had done a stint in Kaback’s genetics department and had been over to Kaback’s house for dinner. Baby Michael appeared to be regressing, and Zeiger asked Kaback to examine him. Kaback had the unenviable task of informing Zeiger that the baby had Tay-Sachs, which is more common among Ashkenazi Jews than the general population. “It was a devastating disease and an enormously powerful experience as a young doctor,” says Kaback, who at 78 is retired from the University of California, San Diego, School of Medicine.

Tay-Sachs is a stealth disease. Newborns develop on a perfectly normal trajectory for the first several months of their lives, doing the yeoman’s work of being a baby: neurons firing, neck and core muscles strengthening, eyes exploring the canvas of the world. But by six months, if they could previously sit up, they no longer can. Their eyes begin to wander and by ten months or so, they suffer convulsions and go downhill day by day. They lose their sight. By eighteen months, they’re in an almost chronic vegetative state, bedridden and unable to do much of anything. “It’s a horror to watch a child deteriorate like that and not be able to do anything,” says Kaback.
When Michael was diagnosed, Karen was well into her second pregnancy. The chairman of Kaback’s department thought the Zeigers should have a test to find out whether their fetus had Tay-Sachs, but Kaback disagreed and felt that they should wait until the baby was born. “I was concerned that if Karen found out that she was going to have a second Tay-Sachs baby, she might jump off the roof.”
After much deliberation, the couple chose not to test. Instead, they decided they would not see their infant until after it was born and had been declared healthy. They couldn’t bear to parent two dying children. If their second baby was found to have Tay-Sachs, they would place the newborn in a home care facility or foster care. They would never lay eyes on their child. It was an agonizing decision born of emotional self-preservation, as Kaback recalls, and it presented him with a dilemma. At the time, Tay-Sachs could be detected only in utero or in a baby who had begun to show symptoms. Whether the disease could be diagnosed in an asymptomatic newborn was unknown. It was likely, but had never before been done. Kaback, who had grown close with the Zeigers, knew he had little choice but to do his best to find out.
He began collecting cord blood from healthy newborns to build a bank of control samples so he would be able to compare them to blood samples from babies with Tay-Sachs. In addition, Kaback reached out to John O’Brien at the University of California, San Diego, who in 1969 had discovered the missing enzyme that fuels Tay-Sachs, and Edwin Kolodny at the National Institutes of Health (NIH), who had also helped identify the genetic cause behind the disease. He told them he’d send them control samples in advance of the Zeiger baby’s birth; after the baby was born, he’d quickly send them the newborn’s cord blood. Separately but together, the three would try to reach a diagnosis.
The last weeks of Karen’s pregnancy raced by. Kaback was in the room when she delivered a baby girl. The moment a child is born is usually one of transcendence, but this time was different. Kaback recalls how Karen and Bob each covered their eyes because they didn’t want to know the baby’s gender. Kaback collected the cord blood sample and raced to his lab. It was 2:00 p.m. He divided the sample into thirds — for O’Brien, for Kolodny, and for himself. By then it was 4:00 p.m. There was a direct flight to San Diego from Friendship Airport (now Baltimore–Washington International), between Kaback’s lab in Baltimore and Washington, D.C. This was long before airport security guidelines made it all but impossible for non-passengers to access airport terminals. Kaback sped to the gate for the San Diego flight and gave the Zeiger baby’s blood sample, nestled in dry ice, to a flight attendant.
“I said, ‘There’s a baby’s life in flux and there’s a handsome doctor who will meet you in San Diego. Will you take it?’” “Of course,” she responded.
Kaback called O’Brien to tell him to meet the plane on the opposite coast, then drove directly to Washington to hand-deliver the second sample to Kolodny at the NIH.
The baby’s blood held the key to detecting Tay-Sachs, even in a newborn who appeared, by any standards, to be perfectly healthy. Tay-Sachs is caused by a deficiency of hexosaminidase A (Hex-A), an enzyme responsible for degrading fatty-like substances in the nervous system. Without it, the fatty substances aren’t broken down, and they build up in nerve cells. This results in the central nervous system disintegrating; children become completely incapacitated and typically die before starting kindergarten.
In 1970, it was not yet possible to sequence a baby’s genome. Kaback could only test for the presence of the enzyme. If there was no Hex-A, the baby had Tay-Sachs.
On the day that Karen gave birth, Kaback worked late in his lab at Hopkins, running Hex-A tests over and over. As the clock ticked toward 2:00 a.m. Kaback had run the Hex-A tests eight times, in three different ways, to be certain. Every result had returned high levels of the enzyme. It was a reassuring outcome. Kaback picked up the phone and called O’Brien, who had met the plane around dinnertime in California. Both O’Brien in San Diego and Kolodny at the NIH achieved similar results. “Everyone, using different methods, found loads of Hex-A,” says Kaback. The baby did not have Tay-Sachs.
Bob hugged Kaback. He and Karen still faced the loss of their firstborn; Michael would die before his third birthday. But their new baby, a daughter whom they would name Joanna, would grow up to get her master’s in genetic counseling and her Ph.D. in genetic epidemiology, and would place fourth in the triathlon competition at the 2000 Olympics.
Together the two physicians walked to the far end of the Johns Hopkins Hospital. First, Bob went to Karen’s room in the obstetrics ward and told her the good news. It was 5:30 a.m. As fate would have it, Bob was on rotation as an intern covering the nursery, so when Joanna was born, she had been cared for in the pediatrics ward in a different part of the building. Karen got out of bed and went to the pediatrics ward with Bob, where they held their healthy daughter for the first time.
Kaback left to go home to process the power of what had just unfolded. It was possible to detect affected fetuses via amniocentesis, but if carriers could be identified before they conceived, or before a pregnancy was advanced, parents could make informed decisions about having children. They could be in control.
•
Detecting carriers in the Ashkenazi population was a daunting prospect. There was no precedent. No one was screening entire populations for genetic diseases. “There was no population-wide carrier screening, period,” says Kaback. A framework would have to be created from the ground up. Kaback decided this would be his life’s work, to establish population-based carrier screening in the Jewish community. The task would require a massive commitment of money, time and education. “Most doctors in practice had never heard of Tay-Sachs,” says Kaback. “The first thing that Mrs. Rosen is going to do when she hears about screening is pick up the phone and call her doctor and her doctor will say, ‘What are these crazy Ivory Tower people saying?’”
In Washington, Harold Gershowitz, who had a child with Tay-Sachs, called a meeting. Kaback showed slides depicting where Jews with the genetic mutation had come from and how it was possible, by identifying carriers of the mutation, to prevent this killer of a disease. He also put up slides of Gershowitz’s son Steven’s development and steady decline.
The effort would need supplies, equipment and personnel. “Harold said he would raise the funds. I showed my slides, I left, and Harold called that night to say he had raised $85,000.”
The plan was to enlist the support and infrastructure of the Jewish community to host screenings at synagogues and community centers. Kaback, a secular Jew, secured the support of Baltimore area rabbis. Press conferences were scheduled, the word went out, and people started calling, eager to sign up. In 1971, the year after Joanna Zeiger was born, 11,000 people were tested. The first screening took place at a Bethesda synagogue on a rainy Sunday afternoon. Fifteen doctors had volunteered to draw blood; 1,800 people showed up to be screened, and Kaback was ecstatic. “It’s like you’d written a symphony, never heard it played, [and] then suddenly it was played and it was perfect,” says Kaback.
News of the ambitious program spread. Doctors came from Canada, from England, from Mexico, to observe and return to their home countries to set up something similar. Screening programs were established in Australia, in South Africa, in South America, in Europe. Kaback was invited to advise many of these countries, including Israel. A country of Jews had a particular need for a systematic way to screen would-be parents. O’Brien’s Hex-A blood test was modified and automated, which made it possible to accurately screen large numbers of people in a short period of time.
Yet opposition came from an unexpected source: the Jewish community itself. A representative of Hadassah, a Jewish women’s organization that had been active in helping organize the first screenings in Baltimore, wrote an essay expressing her concern that the Tay-Sachs screening program was stigmatizing Jews, saddling them with the specter of “bad genes.” What followed was a meeting at the National Institutes of Health that included the director, Kaback, and members of the Jewish community. Kaback made the case that screening wasn’t about eugenics; it was about information. The screening program carried on.
•
What started with the birth of one baby in a hospital in Baltimore has grown to become a prototype for how grassroots organization, public education, physician buy-in, genetic counseling, and genetic technologies can combine to nearly extinguish a disease in a population. From Joanna Zeiger’s birth until 2010, more than 50,000 carriers were identified, among them more than 1,500 couples. As of 2006, when Kaback retired, he had logged 700 Tay-Sachs pregnancies that had been electively terminated due to the pairing of screening with prenatal diagnosis. “Most importantly, more than 2,800 healthy unaffected offspring have been born to these at-risk couples,” Kaback wrote.
Indeed, the ethical considerations were fairly easily dispatched: few could argue that it was a good thing to bring a child into the world whose brief life would be marked by suffering and inexorable, fatal decline.
The success of carrier screening depends upon its uptake. Within the Jewish community, screening programs have reduced the incidence of Tay-Sachs by 95% in the United States and Canada. But there are other communities that have similar carrier rates — French-Canadians and Louisiana Cajuns, for example — in which the importance of screening has not been trumpeted as loudly. Even as a combination of vigilance, dedication, and education has slashed the Tay-Sachs rate among Jews, babies in other communities continue to be born with this terrible disease. Could screening every woman during, or before, pregnancy be the solution?
As a disease, Tay-Sachs met critical criteria for the establishment of a major screening campaign: it was serious — so serious that it was untreatable — and detectable within a defined population. A simple, accurate, and relatively inexpensive carrier test existed, making it possible for parents to know their risk of conceiving an affected child. And it was possible to identify and prevent births through the use of prenatal diagnosis and, if desired, abortion. But even Kaback, who was instrumental in making Tay-Sachs the first widely screened-for genetic disease, isn’t sure that screening far and wide for multiple diseases makes sense.
As president of the American Society for Human Genetics in 1991, Kaback was resistant to universal carrier screening for cystic fibrosis, which is life-limiting but not typically fatal in childhood. “At that point in time, we had a newish screening test, a major mutation had just been identified, and people were talking about mass screening right away,” says Kaback, who favored waiting until the test had proved itself. “Are we going to abort fetuses because they can’t run a marathon?” says Kaback. “You get into complicated issues of quality of life.” For further reference, he cites Gaucher disease. It’s part of an extended panel of tests for Jewish genetic diseases that is routinely recommended to Jewish women in major metropolitan areas, and with apparent good reason: Gaucher disease, type 1 (there are three subtypes) is far more common than Tay-Sachs. It affects about 1 in 450 Ashkenazi Jews, and the carrier rate in this community is a sky-high 1 in 10. Yet the severity of a disease such as Gaucher type 1, which can cause fatigue, anemia, bruising, bleeding, and severe bone pain, doesn’t begin to approach the magnitude of Tay-Sachs. “Gaucher doesn’t belong in there,” says Kaback, who says that its effects can be so mild that there can be “zero symptoms for fifty years.” The average age of initial symptoms in the Ashkenazi population — an ache in a hip or a little bruising — is 45. Is it right to screen for a disease that isn’t fatal?
The Jewish community’s response to Tay-Sachs may be a public health success story, and yet it has led to little standardization in carrier screening, even among Jews. The genetic testing guidelines from the major professional organizations vary considerably from the testing recommended in metropolitan areas with large concentrations of Jews. The American College of Obstetricians and Gynecologists (ACOG) recommends that Ashkenazi Jews be screened for Tay-Sachs disease, Canavan disease, cystic fibrosis, and familial dysautonomia, and the American College of Medical Genetics and Genomics (ACMG) adds five more diseases. But labs that focus on Jewish genetic diseases screen for about 19 disorders and as many as 38, with the number continuing to rise.
•
Peter Kasdan is trying to change the tests through sheer strength of will. In his first pulpit after he graduated from rabbinical school, Kasdan taught a high school girl with Gaucher disease. “She was always sick,” he says. “We always worried about her.” As a clergyman, Kasdan knew better than most about comforting the sick. What he didn’t know at that time was that having a child with Gaucher — or other diseases that disproportionately affect Jews — could be avoided. He learned that several years later, in 1975, at the national convention of the Central Conference of American Rabbis, the professional organization for Reform rabbis in North America. During the conference, a resolution was passed to “urge those couples seeking their officiating at marriage ceremonies to undergo screening.”
Kasdan was then in his fourth year as spiritual leader of the Reform congregation Temple Emanu-El in Livingston, New Jersey. A self-described type-A personality, he took the resolution to heart, and went a step further. When couples asked him to perform their wedding ceremony, he wouldn’t just encourage them. His approach was: “I’m not going to urge them. I’m going to tell them: ‘You want me to do your wedding? Get tested.’”
I don’t know of other rabbis who refuse to offociate at a marriage if a couple rejects testing, but Kasdan’s success rate is impressive. Now retired at 75, he has officiated at more than 1,000 weddings. Only two couples declined testing; Kasdan, in turn, declined to marry them. If couples who get tested learn they’re both carriers for the same disease, Kasdan, along with a genetic counselor, helps them work through the issues.

Image by Dov Pinker
The options, as he sees them: decide not to have kids; choose to adopt; create embryos in a fertility clinic and discard those that have the disease; or get pregnant the regular way, test the fetus, and abort if disease is confirmed. Some couples, he acknowledges, may decide to continue an affected pregnancy. “I have couples who have said to me that it’s God’s will,” he says. “I believe that God has given human beings free will. I would never attribute blame to God if parents knowingly give birth to a child with a fatal Jewish genetic disease. Do they really have the right to give birth to a child whose body will self-destruct? To me, it’s a moral issue. But ultimately that will not stop me from standing under the chuppah with them.”
Kasdan’s determination has contributed to a significant shift in thinking about the burden of inherited disease. He hopes to bring the same public awareness that Tay-Sachs has achieved to other genetic diseases — including Canavan disease, which killed two of his tiny congregants at ages 2 and 4 — that disproportionately affect Ashkenazi Jews. He is fighting no less powerful an entity than ACOG, the professional organization for the nation’s more than 30,000 ob-gyns, which has historically supported carrier screening primarily for diseases whose impact is severe and first observed early in life. While a disease such as Tay-Sachs certainly meets that standard, that’s not the case, as Michael Kaback has noted, for a disease such as Gaucher.
To build his case and educate doctors about the importance of expanding testing, Kasdan, rabbinic advisor to the Jewish Genetic Disease Consortium, leads a medical Grand Rounds program in which geneticists and genetic counselors travel to hospitals to teach obstetricians and pediatricians about hereditary Jewish genetic diseases. He also addresses graduating classes of rabbinical students of all denominations, advising freshly minted rabbis to connect with a local genetic counselor wherever they land a congregation. He delivers his spiel about the importance of catching hereditary disease before it takes its toll on yet another generation. “I have no patience for rabbis or doctors who know the reality and don’t do anything about it,” Kasdan says.
While Kasdan is not advocating that a couple who learns they are both carriers scuttle their impending marriage, Dor Yeshorim, an organization that was founded by an Orthodox rabbi who lost several children to Tay-Sachs, is recommending that they think twice. Dor Yeshorim, based in New York City, maintains an anonymous database of genetic testing results. Potential mates — mostly Orthodox Jews — reference the database for what Dor Yeshorim calls a “compatibility check,” preferably before “a couple or the parents meet, to avoid unnecessary heartache.” If they’re found to both be carriers for the same disease, they are “informed that the match is incompatible.” Couples insistent on pursuing a relationship are offered genetic counseling.
•
Turning pre-pregnancy carrier screening into the new norm would require nothing short of a transformation in the way pregnant women access care. “I have spent my whole life introducing tests, and doctors don’t understand their value and insurance companies won’t pay for them,” says Arthur Beaudet, a Baylor College of Medicine geneticist. Still, he foresees a future, just a generation away, where this transformation will no longer be revolutionary. Classic carrier screening — picking and choosing which diseases to screen for — will simply be rolled into far-more-comprehensive DNA-sequencing tests. “By the time my children are having their own children, Beaudet told me, “everyone will do it.”
Bonnie Rochman is an award-winning journalist. A former health and parenting columnist for Time.com, she has written for The Wall Street Journal, Scientific American and O, The Oprah Magazine. Follow her on Twitter, @brochman
Excerpted from “The Gene Machine: How Genetic Technologies Are Changing the Way We Have Kids — And the Kids We Have.” Copyright © 2017, published by Scientific American / Farrar, Straus and Giroux.
A message from our Publisher & CEO Rachel Fishman Feddersen

I hope you appreciated this article. Before you go, I’d like to ask you to please support the Forward’s award-winning, nonprofit journalism so that we can be prepared for whatever news 2025 brings.
At a time when other newsrooms are closing or cutting back, the Forward has removed its paywall and invested additional resources to report on the ground from Israel and around the U.S. on the impact of the war, rising antisemitism and polarized discourse.
Readers like you make it all possible. Support our work by becoming a Forward Member and connect with our journalism and your community.
— Rachel Fishman Feddersen, Publisher and CEO